Call for Collaboration:Research on the Plasticity Mechanisms of Brain-Computer Interfaces Based on the DIKWP Artificial Consciousness Model
World Academy for Artificial Consciousness (WAAC)
International Standardization Committee of Networked DIKWP for Artificial Intelligence Evaluation(DIKWP-SC)
World Artificial Consciousness CIC(WAC)
World Conference on Artificial Consciousness(WCAC)
Email: duanyucong@hotmail.com
Directory
Research content and key innovations
Technical roadmap and system architecture diagram
Task breakdown with a three-year timetable
Pseudocode and key module design instructions
Team composition and division of responsibilities
Risk assessment and response strategies
Budget structure and description of funding
Expected outcomes and societal value
Project Background
Post-stroke motor and cognitive dysfunction and intractable seizures are two major neurological problems that pose a serious threat to human health worldwide. Stroke (stroke) is one of the second leading causes of death and disability worldwide, with tens of millions of new cases each year. In China, the survival rate of stroke patients continues to improve but the disability rate remains high, and millions of stroke survivors have long endured the difficulties and social burdens caused by hemiplegia, aphasia and other functional impairments. Traditional rehabilitation treatment is often time-consuming and has limited effect, and many patients are unable to fully regain their ability to take care of themselves in daily life, and new interventions are urgently needed to promote brain restructuring and functional reconstruction.
At the same time, epilepsy affects about 50 million patients worldwide, of which about 30% are drug-refractory epilepsy, and frequent seizures bring great risks to patients' cognitive function, mental health and life safety. Existing medical therapy does not cover all patients, and surgical resection of the lesion is only available in a small number of cases. Therapies such as implantable vagus nerve stimulation (VNS) and responsive nerve stimulation (RNS) that have emerged in recent years have brought new hope to intractable epilepsy. For example, closed-response neurostimulation devices (e.g., the Neuropace RNS) can automatically discharge to suppress seizures when abnormal brain electrical activity is detected, and long-term follow-up has shown that they reduce the frequency of seizures by about 60% on average. However, even with the above progress, seizures cannot be cured, and the mechanism of network damage and plasticity changes in the brain under the action of continuous epileptiform discharge has not been fully elucidated, and more in-depth mechanistic studies and more intelligent intervention programs are needed.
Brain-Computer Interface (BCI), as a direct bridge between the brain and external devices, has shown great potential in the field of neurorehabilitation and brain disease intervention in recent years. It is able to decode the patient's brain signals into instructions to control external devices or feedback stimuli, enabling "mind manipulation" and functional compensation. For example, for patients with hemiplegia after stroke, a BCI-based rehabilitation system can identify the patient's residual motor purpose, drive a mechanical exoskeleton or functional electrical stimulation device for the paralyzed limb, and realize motor feedback training synchronized with brain activity. This brain-controlled motor feedback can enhance brain reorganization through the mechanism of "use and use, and retreat", and it has been shown that BCI combined with electrical stimulation training can significantly promote the recovery of motor function in patients with chronic stroke, and maintain the efficacy half a year after the end of treatment. Correspondingly, in the field of epilepsy, closed-loop brain-computer technology also shows the promise of inducing changes in brain plasticity and reducing seizures. It has been hypothesized that targeted electrical stimulation can be given in a non-seizure, low-risk brain state in a non-seizure state that can be used to "reshape" the epilepsy network in a healthy direction, rather than giving stimulation during a seizure. Recent reports support this hypothesis: a more significant reduction in seizure frequency occurs when the closed-loop device is stimulated more frequently during periods of relative calm, suggesting that time-specific neuromodulation may achieve long-term suppression of epilepsy through plasticity alterations.
Although BCI has brought new ideas for stroke rehabilitation and epilepsy intervention, most current BCI systems still have the problem of insufficient intelligence. On the one hand, traditional BCI is mostly a one-way pathway, either decoding brain signals to drive external devices (for rehabilitation training) or detecting abnormal activities to trigger stimuli (for disease suppression), lacking deep two-way communication with the brain. On the other hand, the decision-making of the existing closed-loop system mainly relies on pre-set algorithms or simple rules, and lacks the understanding of the high-level semantic state of the brain and the patient's purpose goal. For example, conventional RNS devices for epilepsy only monitor EEG threshold-triggered stimuli and cannot adjust strategies based on the patient's state of consciousness at the time or long-term cognitive effects; Similarly, BCI for stroke rehabilitation rarely considers the influence of factors such as attention and motivation on the training effect. This "shallow" closed loop is difficult to adapt to individual differences and dynamic changes in brain state, which limits the improvement of efficacy.
At the same time, the field of artificial intelligence is undergoing a paradigm shift from data-driven to "intelligent self-knowledge". The artificial consciousness model provides a new opportunity for the realization of autonomous cognition and decision-making of machines. In this direction, the "DIKWP" network cognition model proposed by Prof. Yucong Duan's team has attracted extensive attention. The DIKWP model introduces the highest level of Purpose (Purpose) on top of the classic "Data-Information-Knowledge-Wisdom" cognitive level**, so that the agent has a clear goal orientation. The model realizes two-way feedback of semantics at all levels through mesh interaction, and gradually improves the perceived raw data to knowledge and wisdom, and the embedded Purpose unit continuously calibrates the decision according to the goal. This mechanism makes every step of AI's reasoning traceable, and the decision-making process becomes explainable and controllable for humans. It is foreseeable that the integration of artificial consciousness models such as DIKWP into closed-loop brain-computer interface systems is expected to give them "adaptive wisdom": the system is no longer only based on superficial signals to respond mechanically, but can understand the purpose of rehabilitation or treatment**to optimize the human-computer interaction strategy from the overall perspective. For example, in stroke rehabilitation, artificial consciousness can realize that the ultimate goal of training is to restore the patient's specific limb function, so as to dynamically adjust the stimulus and task difficulty; In epilepsy intervention, artificial consciousness can intelligently select the timing and intensity of stimulation with the purpose of reducing the seizure rate without impairing cognition. This kind of design that introduces high-level human cognition into the BCI closed-loop is expected to greatly improve the system's adaptability to complex physiological states and the driving effect of functional reconstruction.
In conclusion, this project is based on the frontier interdisciplinary field of "brain-computer interface-induced brain plasticity mechanism", and proposes a new two-way closed-loop BCI framework integrating the DIKWP artificial consciousness model in response to the major needs of stroke rehabilitation and epilepsy intervention. By focusing on both basic mechanism research and engineering implementation, we plan to elucidate the scientific principles of brain remodeling with the participation of artificial intelligence, overcome the key technical bottlenecks of intelligent neuromodulation, and finally build a prototype system that can be used in stroke and epilepsy populations, opening up a new path for the rehabilitation treatment of major diseases.
Core scientific questions
In this context, the project will focus on the following core scientific questions:
Mechanism of brain network plasticity: What neural circuit reorganization and brain network plasticity changes are involved in the recovery of brain perception and motor function and the improvement of brain disease symptoms under the long-term effect of brain-computer interface? For example, how can residual neural circuits be strengthened or reorganized through training during the reconstruction of the function of a paralyzed limb after a stroke? How does closed-loop stimulation induce the transition of abnormal discharge networks to a healthy model in epilepsy patients? These plastic changes cover the multi-level mechanisms from synaptic strength adjustment to functional network topological reorganization, which are still not well understood and need to be further studied and elucidated.
Coupling of consciousness and cognitive networks: In dysfunctional states such as dysfunction or seizures caused by stroke, how is the normal whole-brain network coupling and information integration process disrupted? When consciousness and cognitive function are impaired, how do the connection patterns of the various functional areas of the brain change? This project will explore the relationship between whole-brain network coupling mechanism and consciousness/cognitive state, and analyze which key networks (such as default network, attentional control network, etc.) are weakened or abnormally synchronized in the state of cognitive impairment. Based on this, we further explore how the introduction of the DIKWP artificial consciousness model can drive the reconstruction and functional improvement of the brain's cognitive network: can artificial consciousness, as an external agent, recoordinate the dysfunctional network connection through interaction with the brain, and promote the recovery of patients' cognitive ability and consciousness level?
DIKWP Closed-Loop Cognitive Control: How should agents based on the DIKWP artificial consciousness model form a two-way closed-loop interaction with the patient's brain to achieve intelligent regulation of neural activity? Specifically, how can artificial consciousness obtain semantic information about the patient's state from multimodal brain signals and generate optimal intervention decisions through the five-layer reasoning loop of "data-information-knowledge-wisdom-purpose"? How does the closed-loop system have the ability to adapt to learning and continuously adjust strategies based on long-term efficacy feedback? Especially in the pathological process of dynamic changes such as epilepsy, how to ensure the real-time, stability and safety of artificial consciousness decision-making, and realize the timely and robust control of abnormal neural activity?
Multi-objective synergy and predictability: In practice, rehabilitation or treatment often involves multiple goals. For example, stroke rehabilitation needs to improve motor performance while taking into account cognitive and language functions. Epilepsy interventions are both to reduce seizures and avoid negative effects on cognitive functions such as memory. Can changes in brain function be predicted and quantified with the intervention of artificial consciousness? How can closed-loop intervention achieve multi-objective collaborative optimization and avoid "taking care of one at the expense of the other"? We will study and establish corresponding models and index systems to predict the functional evolution trajectory and potential side effects after artificial consciousness intervention, and guide the system to achieve the main goal without introducing new functional deficits. The mode of action and interaction mechanism in the process of multi-objective optimization are also important scientific issues, which need to be clarified through simulation and experiments.
Chip implementation and system integration: How to effectively implement the DIKWP artificial consciousness model into a clinically usable implantable**/non-implantable hardware system**? At present, there is no mature device of "artificial consciousness + brain-computer interface" for reference, and this project needs to develop a prototype from scratch. What engineering and technical difficulties need to be overcome for this? For example, real-time neural signal processing, low-power and high-efficiency AI chip design, biomaterials and safety isolation, etc. How can the entire system architecture be designed to take into account the strict constraints of size and power consumption, while ensuring that wireless communication, biocompatibility, and safety protection meet clinical requirements? This question is related to the feasibility of transforming research results into practical applications, and we will explore them in depth in the project.
The above scientific questions are interconnected and cover the entire chain from basic theory to technical realization. By addressing these key issues, this project will provide new perspectives for understanding and harnessing brain plasticity, and lay the theoretical and technical foundation for a new generation of intelligent brain-computer interface systems.
Research Objectives:
The overall goal of this project is to elucidate the plasticity mechanism of brain function remodeling under brain-computer interface conditions, and to create a new closed-loop brain-computer system method integrating the DIKWP artificial consciousness model to improve the effectiveness of stroke rehabilitation and epilepsy intervention. With this overarching goal in mind, we have established the following specific goals:
Unraveling the neuroplasticity induced by brain-computer interfaces: Through animal models, imaging, and electrophysiology, we quantitatively study the recombination changes of brain neural circuits and functional networks under closed-loop brain-computer stimulation or training, and elucidate the key plasticity mechanisms that promote motor function recovery and inhibit seizures.
Construct a whole-brain network model in the state of consciousness disorder: establish a model that reflects the multi-region coupling characteristics of the whole brain in the state of brain dysfunction and seizures after stroke. To describe the dynamic characteristics of the network when consciousness and cognitive function are impaired, and to provide a targeted basis for artificial consciousness intervention.
Development of DIKWP-driven closed-loop control algorithm: Design and implement a closed-loop brain-computer intelligent control algorithm based on the DIKWP artificial consciousness model, including multi-level neural signal semantic decoding, purpose-driven decision-making engine and adaptive learning strategy, so that the system can generate safe and effective intervention instructions in real time and continuously optimize.
Build a two-way brain-computer interaction simulation platform: Establish DIKWP×DIKWP mesh interaction models to reproduce the two-way communication process between artificial consciousness and biological brain in the simulated environment, verify the effectiveness of multi-objective reasoning, semantic communication, adaptive feedback and other mechanisms, and provide design reference for actual systems.
Development of a closed-loop brain-computer interface prototype system: Integrate core modules such as artificial consciousness processing chips, cognitive feedback engines, and semantic perception decoding, and develop two-way closed-loop brain-computer interface prototypes that can be used for animal experiments and even preliminary clinical tests. The system needs to verify the functional effect and safety in stroke rehabilitation training and seizure intervention, and lay a foundation for subsequent clinical application.
Through the realization of the above goals, we hope to deepen the understanding of brain plasticity and intelligent intervention in science, create a new brain-computer interface paradigm led by artificial consciousness in technology, and promote the leapfrog progress of brain disease rehabilitation technology.
Research content and key innovations
Focusing on the research objectives, we have planned the following four major research contents, each of which condenses specific scientific/technical questions and innovations:
1. Research on the mechanism of neural circuitry and network plasticity under brain-computer interface This
section studies the plasticity changes that occur in the brain during the reconstruction of motor/sensory function and the improvement of seizures after stroke. The main contents include:
Neural circuit reorganization in stroke rehabilitation: Stroke model animals or convalescent patients were recruited to observe the changes in brain functional connectivity before and after BCI feedback training by functional magnetic resonance imaging (fMRI), transcranial magnetic stimulation (TMS) and other techniques. It focuses on the remodeling of motor control-related networks (such as cortical-cortical connections and cortico-spinal pathways), and quantitatively evaluates the evolution of brain network topology from injury state to recombination enhancement. For example, resting-state fMRI was used to analyze changes in the strength of functional connectivity in brain regions to verify whether BCI training enhanced functional connectivity between impaired hemisphere motor regions. By comparing different training programs, the effects of sensory feedback and visual feedback on plasticity effects were discussed, and how the perceptual-motor closed loop could synergistically promote neural circuit reconstruction was revealed.
Network plasticity in epilepsy interventions: To investigate the mechanism of long-term closed-loop stimulation on the modification of abnormal discharge networks in patients with refractory epilepsy monitored by animal models of epilepsy (e.g., rodent ignition models) or implanted electrodes. The evolution of EEG network characteristics (such as synchronization rate and graph index) before and after the seizure was recorded by high-density electroencephalography (ECoG/EEG) and magnetoencephalography (MEG), and whether closed-loop stimulation reduced the oversynchronization between the lesion and other regions of the whole brain, and whether it induced the formation of new inhibitory connections, thereby improving network stability. Combined with molecular biology assays, such as neurotransmitter receptor expression changes, to investigate the role of electrical stimulation-induced synaptic plasticity (LTP/LTD) in network remodeling. This part of the innovation lies in the introduction of brain network science into the evaluation of epilepsy stimulation therapy, most previous studies have only focused on changes in seizure frequency, and we will quantitatively describe for the first time how closed-loop interventions can change the network topology of the epileptic brain, providing a basis for understanding its long-lasting efficacy.
Innovation: Combining neuroimaging, EEG network analysis and BCI intervention, the system reveals the influence mechanism of closed-loop brain-computer interaction on neural circuit and network plasticity under two different pathologies: stroke and epilepsy. This can not only fill the gap in the current understanding of the mechanism of brain stimulation and plasticization, but also provide a biological target for optimizing intervention strategies.
2. Unconscious-Cognitive Whole Brain Network Coupling and DIKWP Cognitive Improvement Mechanism in Dysfunction This
section focuses on the whole brain network coupling and cognitive mechanism in stroke and epilepsy patients in the state of functional impairment, and explores how artificial consciousness intervention can promote network reconstruction and cognitive improvement
Network representation of consciousness/cognitive dysfunction: Brain network analysis is used to characterize the whole brain network characteristics of patients with severe stroke (who may have comorbid impaired consciousness such as coma/semi-comatose state) and epilepsy patients (especially when status epilepticus or frequent seizures lead to confusion). For example, the whole brain functional connectivity density, small world coefficient and other indicators are calculated to compare the differences between patients and healthy people. It is expected that the destruction of the modular structure of the brain network and the weakening of the function of key knots can be observed, reflecting the decrease in the coordination of various brain modules when consciousness and higher cognitive processes are impaired. Furthermore, combined with the frequency domain analysis of EEG/magnetic encephalography signals, typical signs such as slow wave enhancement and anterior-posterior regional desynchronization when the level of consciousness decreases were studied, and the network criterion of the degree of consciousness impairment was established.
DIKWP Artificial Consciousness-Driven Network Reconstruction: Based on the above understanding of pathological networks, we will design simulations and experiments to verify the impact of DIKWP artificial consciousness model intervention on brain network coupling. For example, a compromised network (calibrated based on real patient data) is simulated in a computer model and the DIKWP agent interacts with it: the agent reads the network state and generates an intervention to promote network connectivity through its "purpose" module (e.g., simulating a node or asking the patient to perform a specific cognitive task) to see if the network metrics improve. This is similar to using artificial consciousness as an external "brain" to form a DIKWP ×DIKWP network dialogue with the patient's brain. We will focus on whether the overall efficiency and information integration can be improved under the effect of artificial awareness. In this process, if possible, we also plan to validate in the trials in which patients participate: having stroke/epilepsy patients receive BCI training with cognitive feedback (e.g., requiring concentration to complete BCI tasks, and artificial awareness modules give prompts or adjust the difficulty of the task according to their brain signals) to observe changes in the patient's brain network and the improvement of cognitive function. It is expected that artificial consciousness can restart or strengthen cognitive-related connections in the patient's brain, such as improving the synergy between the prefrontal lobe and other regions, leading to an increase in cognitive test scores.
Key Innovation: Introducing Artificial Consciousness into the Intervention of Cognitive Impairment in Brain Diseases, which is a new research paradigm. We innovatively put forward the concept of "whole-brain network coupling reconstruction", emphasizing not only the treatment of local functions, but also the restoration of the overall information integration ability of the brain. The DIKWP model gives the system the ability to understand the patient's high-level purpose and cognitive state, which is different from the traditional passive way of simply stimulating neural circuits, and embodies the concept of closed-loop cognitive control, that is, to promote recovery by understanding and guiding the patient's conscious activities. This mechanism of human-computer collaborative gain is an original exploration of this project, which is expected to expand the application boundaries of brain-computer interface.
3. Simulation of Bidirectional Closed-loop Brain-Computer System Based on DIKWP×DIKWP Interaction ModelIn
this part, we will establish a mathematical model and simulation platform to verify the key processes of artificial consciousness and brain interaction, and provide guidance for system implementation. The details are as follows:
DIKWP×DIKWP Mesh Interaction Architecture Design: Drawing on the "dual circulation" artificial consciousness system architecture proposed by Professor Yucong Duan, we build an interaction model that includes the main loop and the meta-loop: the main loop represents the real-time perception and decision-making process of the artificial consciousness to the brain, and the meta-loop represents the monitoring and adjustment of the artificial consciousness to its own decision-making. At the same time, one side of the brain is also abstracted into a DIKWP structure, that is, the brain can also be regarded as a multi-layered flow of "data-information-knowledge-wisdom-purpose" when processing information (where "purpose" corresponds to the intrinsic needs of the organism). Through this symmetrical interaction design, we can study how two DIKWP agents (artificial and biological) can coordinate through semantic level communication. In the model, the brain's "data" comes from neuronal firing, "information/knowledge" corresponds to patterns and memories, "wisdom" corresponds to integrated judgments, and "purpose" is its physiological or behavioral goals. The AI obtains brain data through sensing, and processes it through its own DIKWP layer to form an understanding of the brain state and countermeasures, and its "purpose" is the treatment target. We make the two continuously interact and iterate: the AI makes a stimulus or feedback based on the state of the brain, and the brain changes to generate new signals, and so on. The model will be implemented in the form of a mesh graph, with nodes representing different semantic hierarchical states and edges representing interactions, and we plan to use the model to analyze the stability and convergence conditions of the system, and determine under what circumstances the two sets of DIKWP cycles can achieve synergy and equilibrium.
Multi-objective reasoning and decision-making mechanism: Test the ability of artificial consciousness to deal with multiple objectives in a simulated environment. The setting scenario contains multiple goals (such as improving the patient's motor and language skills at the same time, or controlling two different types of seizures at the same time), and the artificial consciousness is inferred according to the DIKWP model: how to synthesize different goal requirements at the knowledge layer, weigh priorities at the wisdom layer, and finally form a decision that takes into account each goal at the purpose layer. This process can be formalized as a multi-objective optimization problem, but artificial consciousness should be able to approximate the solution through semantic reasoning. We will compare the differences between the artificial awareness strategy and traditional algorithms, such as weighted summation optimization, to see if it can handle goal conflicts more flexibly. In addition, the interpretability of decisions is examined: the DIKWP model unfolds the decision-making process hierarchically, and we will track the intermediate results of each layer to ensure that the contribution of each goal in the decision is clear, so as to achieve the interpretation of multi-objective decisions.
Semantic Communication and Adaptive Feedback: Investigate how the exchange of information between the artificial consciousness and the brain goes beyond simple signaling and rises to "semantic communication". Specifically, we will design protocols that allow artificial consciousness, when interacting with the brain, not only to send fixed stimulus parameters, but also to send signals with semantic markers depending on the context. For example, in rehabilitation, artificial consciousness "informs" the brain about the meaning of current exercises through brain stimulation or sensory feedback (e.g., the purpose of emphasizing a muscle contraction), or conveys semantic cues such as "calm" during relaxation training in people with epilepsy. Our model will simulate how this semantic information is received and decoded by the brain and explore its impact on neuroplasticity. In addition, through the introduction of meta-loops, adaptive feedback control is realized, according to the changes in the brain's response to stimuli, the artificial consciousness adjusts the feedback content and frequency in real time to achieve the best effect. This is similar to an adaptive strategy in reinforcement learning, but is guided by a high-level Purpose in our framework. What's innovative is that we try to give closed-loop systems a certain level of "language" (albeit in the form of neural signals) to explore how machines can communicate with the brain in ways closer to the cognitive level, thereby inspiring more effective malleable change.
Innovation: The core innovation of this part is to propose and validate the new concept of DIKWP ×DIKWP interaction model, and provide a unified description of brain-computer interaction through the dual-agent model. This model allows us to theoretically study complex behaviors such as stability, oscillation, and synergy in the artificial consciousness-brain closed loop. Compared with traditional black-box simulation, we have introduced semantic level considerations, and pioneered human-computer interaction to the level of "understanding and dialogue". In addition, the addition of multi-objective inference and adaptive feedback mechanism makes the system closer to the real application requirements and ensures that the simulation results are of guiding significance for the actual system design.
4. Prototype construction of bidirectional closed-loop brain-computer system and verification of neurointervention effectOn
the basis of the completion of the above basic and model research, this part focuses on engineering implementation and application verification, develops the actual closed-loop brain-computer interface system and evaluates its intervention effect on stroke and epilepsy
- Artificial Consciousness Chip and System Integration: A dedicated DIKWP Artificial Consciousness Processing Unit (ACPU) was developed to solidify the DIKWP model inference algorithm on high-efficiency hardware. The chip design adopts a combined ASIC/FPGA solution: the algorithm function and performance are verified on the FPGA platform, and then the ASIC is designed accordingly to reduce the size and power consumption. The internal architecture of the chip is optimized for the DIKWP model, which realizes parallel processing of data, information, knowledge, and wisdom layers, and adds a Purpose decision-making unit and a metacognitive monitoring unit to support the operation of the "dual-cycle" architecture. In terms of hardware implementation, model pruning, fixed-point operation and other means are adopted to ensure that the chip meets the power consumption and heat dissipation requirements of implantable devices. Then, the ACPU chip is integrated with a multi-channel neural interface to form an implanted bidirectional closed-loop device: including a high-density electrode array (to record brain signals and apply stimuli), a microprocessor module (responsible for communication with the chip and wireless data transmission), a secure power supply and packaging, etc. Biocompatible encapsulation materials and electrode coatings are specially designed to reduce tissue rejection and signal attenuation. The entire device needs to be miniaturized to a position suitable for implantation or wearing in the skull, and wireless connectivity for in vitro monitoring and parameter tuning. The system architecture diagram is shown in Figure 1, which shows the components and information flow of the brain-computer interface prototype
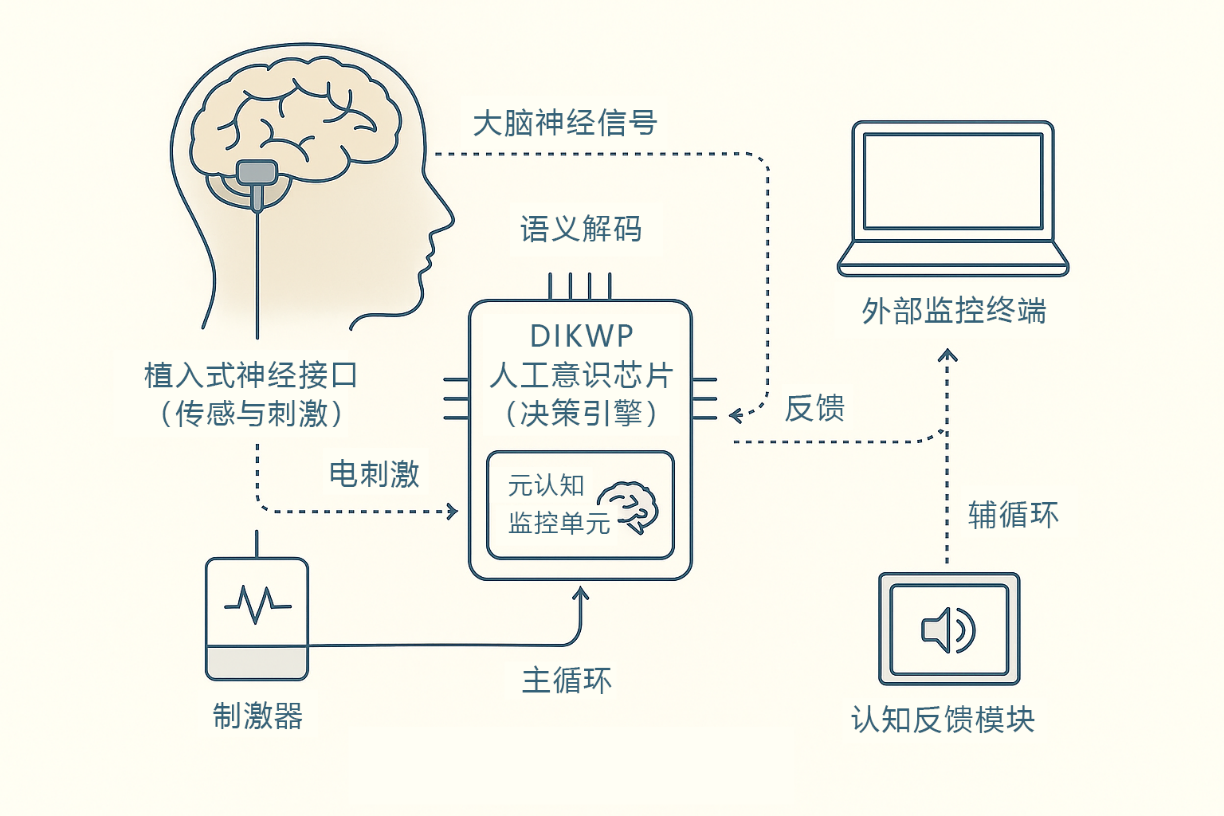
Figure 1 Purpose of the bidirectional closed-loop brain-computer interface system architecture. The system consists of an implantable neural interface (sensing and stimulation), a DIKWP artificial awareness chip (decision engine), a cognitive feedback module and an external monitoring terminal. The brain nerve signals are collected by the sensor and semantically decoded and sent to the artificial consciousness chip for multi-layer inference, and the decision-making instructions generated by the chip drive the stimulator to apply electrical stimulation to specific areas of the brain (closed-loop main circulation) on the one hand, and on the other hand, the feedback module presents the patient in the form of visual/auditory to guide his cognitive state (closed-loop auxiliary circulation). There is a metacognitive monitoring unit inside the artificial consciousness chip, which carries out safety verification and adaptive adjustment of the main cycle decision-making to ensure that the system runs stably and controllably.
Stroke rehabilitation application verification: Qualified stroke hemiplegic patients are selected in the rehabilitation hospital environment, and preclinical experiments are carried out using the developed closed-loop BCI prototype. The brain signal is decoded by the artificial consciousness chip in real time to decode the high-order semantic content such as "Purpose of Movement", and the functional electrical stimulation or exoskeleton drive of the paralyzed limb is triggered after the decision of the Purpose layer, so that the patient can generate actual motor feedback. At the same time, the cognitive feedback module gives encouraging prompts according to the patient's concentration and other indicators, forming a two-way closed-loop training. During the whole process, the changes in the patient's limb motor function score, cerebral cortex excitatory recombination (assessed by TMS evoked potential) and other indicators were monitored. Assuming that this system can significantly improve the training effect compared with traditional rehabilitation methods, we will quantify: for example, compared with the control group, the Fugl-Meyer motor score in the closed-loop group is more improved, and the functional connectivity of the brain network is more significantly restored. Experiments will also verify the safety of the system, such as the absence of infection at the electrode implantation site and the absence of error-free over-excitation of artificial conscious decision-making (meta-monitoring should be avoidable). This will lay the groundwork for large-scale clinical trials in the future.
Epilepsy Intervention Application Validation: To test the effect of closed-loop systems on seizures in clinical monitoring units or animal models of refractory epilepsy. For implanted epilepsy patients, intraoperative or postoperative cortical electrodes are used to connect to the artificial consciousness system: the artificial consciousness chip continuously analyzes the patient's EEG, and when it detects changes in the early epilepsy network, it triggers local electrical stimulation with the purpose of "inhibiting abnormal activities" to try to prevent the spread of seizures. In addition, the system will assess the patient's state of consciousness at that time, and if the patient is awake and anxious, the cognitive feedback module may give soothing instructions to help the patient relax. This combination of hard and soft intervention strategy will evaluate the efficacy during a certain observation period, including changes in the frequency and duration of attacks, and assessment of the patient's cognitive function (to ensure that there are no significant side effects). If animal experiments are conducted, seizures can be induced in animals and closed-loop stimulation intervention can be performed to record the effect of stimulation on neuronal firing and behavioral performance during the seizure. The expected result is that closed-loop artificial consciousness systems are able to reduce the number and intensity of seizures, and even reduce seizure susceptibility after a period of time due to changes in network plasticity. We will also check the reliability of the system, such as the stability of long-term operation, the electrode does not shift and other engineering indicators.
Key Innovations and Values: In this section, we will put the above theories and models into practice to build the world's first closed-loop brain-computer interface prototype that integrates artificial consciousness. Its innovation lies in the integration of a number of self-developed components (DIKWP chip, semantic decoding, cognitive feedback) to form a complete system, realizing the real sense of human-machine integration closed-loop control. Through testing in rehabilitation and disease intervention scenarios, we will validate the effectiveness of this new paradigm and provide direct evidence for future clinical translation. If successful, it means that for the first time, patients can use a "conscious" intelligent BCI system to accelerate brain recovery or inhibit disease progression, which is of epoch-making significance.
Technical roadmap and system architecture diagram
Technical route: This project adopts the progressive technical route of "basic research→ model simulation→ prototype realization →application verification", and each stage is connected and gradually deepened to form a closed-loop R&D process. Firstly, in the basic research stage, we discovered the brain plasticity mechanism and pathological network characteristics through biological experiments and data analysis, which provided a scientific basis for the construction of artificial consciousness control strategies. Then, in the model simulation stage, the DIKWP artificial consciousness-driven control algorithm was developed, and the effect of the algorithm was simulated and verified in the multi-level brain model and the DIKWP interaction model, so as to optimize the algorithm parameters and model assumptions, so that the scheme became mature before being put into hardware implementation. Then, in the engineering implementation stage, the chip and system integration problems were overcome, the prototype of the closed-loop brain-computer interface was developed, and its function was tested on the in vitro bench. Finally, in the application validation stage, the prototype was used for animal experiments and small-sample human trials, closed-loop evaluation of the actual intervention effect and safety, and the research of the feedback and feedback mechanism and algorithm improvement were obtained. This technical route uses theory to guide practice, and uses experimental results to calibrate theoretical assumptions, gradually reduce risks, and ensure the realization of the ultimate goal of the project.
System architecture: Figure 1 shows the overall architecture of the closed-loop brain-computer interface system of this project. The system consists of implanted multi-channel EEG electrodes, DIKWP artificial awareness chip, cognitive feedback module and external control terminal. The workflow is as follows: first, implanted electrodes collect nerve signals (data layers) in the cerebral cortex or deep part, and transmit them to the artificial consciousness chip through signal conditioning; The semantic decoding module built into the chip converts the original signal into useful information and knowledge features (information/knowledge layer), and then the inference engine combines the existing built-in model and the current context to evaluate the brain state in the Wisdom layer. The highest Purpose layer makes decisions based on a stated goal (recovery or suppression of seizures), such as the need to enhance the excitability of a circuit or inhibit abnormal activity. On the one hand, the decision-making signal is sent to the stimulus control module, which drives the implanted electrode to perform electrical stimulation (output to the brain) to the target area. On the other hand, it is sent to a cognitive feedback module and provided to the patient in the form of visual, auditory or tactile perceptions. As a result, patients receive feedback about their own state or training tasks, which generate corresponding cognitive responses in the brain (such as concentration or emotional comfort), which in turn affect brain signals and form a second feedback pathway. The system also contains a metacognitive monitoring unit, which independently monitors the decision output and physiological parameters of the artificial consciousness chip: when an abnormality is detected (such as a decision outside the safety boundary), the parameters can be corrected in real time or the safety mode can be triggered to avoid adverse events. The entire architecture supports two-way information flow: both from the brain to the machine uplink (Purpose decoding) and from the machine to the brain downlink (stimulation and feedback), supplemented by internal self-supervision, to truly achieve a safe and adaptive human-machine closed loop.
The system architecture embodies the research results of the project: it embodies the layer-by-layer processing and purpose guidance of the DIKWP model from the algorithm, and integrates the coordinated operation of sensing, computing, stimulation and feedback components from the engineering perspective. Through the clear display of the architecture diagram, we will guide the actual equipment development accordingly, and ensure that the interface of each module is clear and the functions are complete.
Task breakdown with a three-year timetable
In order to move the project forward, we broke down the research tasks into several modules, which were completed in phases over a period of three years:
Stage 1 (Year 1): The core lies in the basic theory and model preparation. The main achievements are: (1) literature research and pre-experiments related to stroke and epilepsy, collecting neuroimaging and EEG data of disease models, and preliminarily analyzing the differences in brain network characteristics; (2) Establishment and pre-test of animal experiments: establish stroke model mice (such as middle cerebral artery occlusion model) and epilepsy model (such as drug-induced convulsions), carry out preliminary BCI training and closed-loop stimulation intervention experiments, and obtain brain signal and behavior data; (3) Development of the first version of DIKWP artificial consciousness algorithm: realize the basic data → information → knowledge → Wisdom→ Purpose processing link, and adjust the parameters according to the acquired data, so that the algorithm has the ability to recognize simple brain states and give basic decisions; (4) Milestone check: form a stage report for stroke and epilepsy brain network analysis, complete the code verification of the DIKWP algorithm module, and write 1-2 stage papers (focusing on theoretical mechanisms).
Phase 2 (Year 2): The core lies in simulation verification and algorithm optimization. (1) Improve the DIKWP ×DIKWP interaction model framework, use the experimental data of the first year to calibrate the model parameters, carry out multiple sets of simulations (different training schemes, multi-objective scenarios, etc.), and refine the patterns and problems of artificial consciousness and brain interaction; (2) Improve the artificial consciousness decision-making algorithm: introduce reinforcement learning, adaptive filtering and other mechanisms to improve the robustness and real-time performance of the algorithm in complex situations, and add metacognitive monitoring modules to ensure safety; (3) Develop key hardware prototypes: realize artificial awareness algorithms on the FPGA platform, process the first version of multi-channel electrodes and signal acquisition circuits, and test closed-loop performance in an in vitro simulated environment; (4) Continue animal experiments to carry out stroke rehabilitation training and closed-loop stimulation of epilepsy with the improved algorithm to evaluate the improvement of efficacy; (5) Milestone check: Complete the v2.0 version of the DIKWP artificial consciousness algorithm and FPGA verification, obtain the simulation and animal experiment results report, and publish 2-3 papers (focusing on models and algorithms).
Stage 3 (Year 3): The core lies in system integration and application validation. (1) Complete the DIKWP artificial awareness chip (ASIC) design and integrated packaging of tape-out (or advanced FPGA implementation) and implantable closed-loop system; (2) conducting limited clinical testing: testing the safety and preliminary efficacy of the system on volunteer patients (small sample trials with ethical approval) and collecting human data; (3) Comprehensively analyze the results, adjust and optimize the final system parameters and use process; (4) Pre-acceptance results: form a complete closed-loop brain-computer interface prototype system, the function meets expectations, and is verified by bench and animal tests; Write general reports and guideline documents, publish more than 2 high-level papers (focusing on the overall system and experimental effects), apply for relevant invention patents, and prepare for clinical translation.
This schedule ensures that there are clear milestones and outputs each year, while laying the groundwork for subsequent phases. We will conduct an assessment at the end of each phase to ensure that the project is on track and that the plan is adjusted in a timely manner for risk factors (see the Risk Assessment section below).
Pseudocode and key module design instructions
In order to better describe the closed-loop control algorithm based on the DIKWP model, this section provides pseudocode of the main processes and the design description of the key modules.
1. Closed-loop brain-computer control algorithm pseudocode: The following pseudocode illustrates the workflow of the main and meta-loops of artificial consciousness:
Loop forever (each control cycle):
# ==== Main Loop: Data Acquisition & Decision Making ====
raw_data = BrainInterface.acquire_signals() # Get raw data from EEG/neural interface
info = SignalProcessor.extract_features(raw_data) # Extract information features (filtering, eigenvalues, etc.)
knowledge = StateEstimator.interpret(info) # Mapping features to knowledge (judging the current functional state)
wisdom = Evaluator.integrate(knowledge, context) # Synthesize context to form Wisdom (global assessment)
decision = Deliberator.plan(wisdom, Goal) # Develop an intervention plan based on the Purpose of the Goal
# Execute decisions (output to environment and feedback to patients)
Stimulator.apply_stimulation(decision.stim_params) # Stimulates the target area of the brain
FeedbackModule.provide_feedback (decision.semantic) # Provide semantic feedback (e.g. prompts/rewards) to the patient
# ==== Meta-Cycle: Decision Monitoring and Self-Adjustment ====
if Monitor.detect_anomaly(decision, raw_data):
Deliberator.adjust_parameters() # If an abnormality is detected, adjust the internal parameters or enter safe mode
continue # skips the rest of the current loop
# Update your knowledge base and strategy based on long-term feedback
if long_term_feedback_available():
Learner.update_internal_model(long_term_feedback)
In the above pseudocode, each module corresponds to the following functions:
BrainInterface.acquire_signals(): Responsible for interacting with interfaces such as implanted electrodes to read real-time neural signals.
SignalProcessor.extract_features(): Signal preprocessing and feature extraction module, which converts raw data into usable information, such as spectral power, synchronization index, etc., representing the "information" layer of the DIKWP model.
StateEstimator.interpret(): A state assessment module that maps information features to higher-level "knowledge", such as identifying whether the current patient is developing a motor purpose or whether there is a preepileptic discharge pattern.
Evaluator.integrate(): A comprehensive assessment module that integrates multi-source knowledge and historical context at the "Wisdom" layer to make a more comprehensive judgment about the current situation, such as assessing a patient's recovery progress or the level of risk of a recent seizure.
Deliberator.plan(): The unit of decision, corresponding to the "Purpose" layer of the DIKWP. It develops a specific intervention plan based on the system's set goal (e.g., restoring a function, reducing the probability of seizures) and the Wisdom assessment provided by the Evaluator, which includes stimulation parameters, feedback content, etc. Decisions can also carry semantic tags for feedback modules.
Stimulator.apply_stimulation(): Stimulation output module that converts parameters in decision-making into physical stimuli to the brain/peripherals, such as electrical stimulation waveforms, robot movements, etc.
FeedbackModule.provide_feedback(): A cognitive feedback module that translates the purpose of decision-making into a form that is perceived by the patient, such as prompting the patient to "force the image of a fist" through voice, or presenting real-time results on the screen to motivate practice.
The Monitor.detect_anomaly () of the meta-circulation part: The metacognitive monitoring module reviews the output and input signals of the main cycle in real time to determine whether there is any abnormality (such as continuous stimulation does not achieve the expected effect, abnormal physiological indicators of patients, etc.). Once found, execute Deliberator.adjust_parameters () to adjust the internal policy or trigger the security mode, skipping the current cycle to avoid error accumulation.
Learner.update_internal_model(): A self-learning module that updates internal model parameters, such as strategy optimization or model calibration, based on long-term feedback results (e.g., functional assessment after multiple trainings, or changes in seizure frequency after several weeks) to continuously improve system performance.
2. Key module design description:
Multimodal Semantic Decoding Module: This module corresponds to the data → information conversion of the DIKWP model, and is designed to integrate traditional signal processing and deep learning methods. In order to improve robustness, we use multi-modal data fusion: not only EEG/eco-electrical signals can be processed, but also auxiliary information such as electromyography and heart rate can be accessed. Time-frequency features are extracted through convolutional neural networks (CNNs) and classified by semantic tags in the knowledge base (e.g., "trying to move the left hand" for a specific EEG pattern). The information output by the module comes with a preliminary semantic description that can be used for subsequent decision-making. The innovation lies in the introduction of semantic annotation training, so that the low-level signal processing directly serves the high-level meaning interpretation, rather than only extracting statistical features.
DIKWP Decision Engine (Deliberator): This is the "brain" of the system, which implements the core function of artificial consciousness. The design uses a combination of rule-based inference + reinforcement learning optimization: the initial rules are determined by expert knowledge and DIKWP theory, e.g. "If a patient is detected to have a fist Purpose but no actual movement, electrical stimulation is sent"; At the same time, reinforcement learning algorithms are introduced to allow the system to adjust the intensity and timing of stimuli in iterative experiments to optimize long-term returns (such as functional improvement). The decision engine is implemented in five layers corresponding to the DIKWP structure, and the output of each layer is reserved for logging and monitoring to ensure transparency and explainability. For example, when the system decides to increase the intensity of the stimulus, it can be traced back to the knowledge layer that "signs of fatigue have been detected", the Wisdom layer that determines that "the current training effect has decreased", and the Purpose layer increases the intensity accordingly to maintain the target progress. Such a design guarantees interpretable semantics at every step of the decision.
Metacognitive Monitoring & Security Module: This module monitors the operation of the system at all times, independent of the main decision-making process. The implementation adopts a dual-threaded architecture, with one main thread performing the above closed-loop loop and the other monitoring thread performing rule checks on key variables. For example, the stimulus output is monitored for exceeding the safe limit, for abnormally high decision-making frequency, and for dramatic changes in the patient's physiological signals (e.g., heart rate). If any of the safety rules are triggered, the monitoring module immediately takes over: pauses the stimulus output, switches the system to safe mode (e.g. maintains the minimum safe stimulus or stops completely), and alarms the external terminal. The main cycle is resumed only after the parameters are adjusted and confirmed to be correct. This mechanism ensures that there is a second line of defense for patient safety, even in cases where artificial awareness algorithms can go wrong.
Cognitive Feedback Engine: Traditional BCI is often presented with simple visual or auditory feedback, such as cursor movement, success sound, etc. The feedback engine of this system uses the semantic output of DIKWP to design richer interactive feedback. For example, when the patient's attention is distracted and the training effect decreases, the system detects this high-level state, and the feedback engine can voice remind "please concentrate and imagine arm movements"; For example, when a patient is frustrated, soothing music can be played or a message of encouragement can be displayed on the interface. This kind of feedback goes beyond the notification of simple task results and integrates the guidance of the patient's psychological and cognitive state, which helps to mobilize the patient's subjective initiative and form a real human-in-the-loop training. The module needs to work closely with the decision-making engine to select the appropriate feedback strategy based on the output of the Purpose layer to achieve situational adaptation.
In summary, the pseudocode and module design reflect the logical architecture of the software and hardware systems of this project. It can be seen that the DIKWP artificial consciousness model runs through all aspects of data processing, decision-making and feedback, so that the system has the ability to process from low-level signals to high-level semantics, as well as the learning ability from short-term response to long-term adaptation. These designs provide a guarantee for the realization of system functions and the achievement of innovation goals.
Team composition and division of responsibilities
This project is led by the Active Medicine Committee of the World Association of Artificial Consciousness and completed with the collaboration of several units. The project team integrates multidisciplinary experts in artificial intelligence, brain science, clinical medicine, electronic engineering, etc., and the main members and division of labor are as follows:
Prof. Yucong Duan (Project Leader, Supporting Unit: Active Medicine Committee of the World Association of Artificial Consciousness): The general leader and academic leader of this project. He is the proposer of the DIKWP artificial consciousness model and has extensive experience in the field of cognitive computing and brain-computer interface. Responsible for the overall research program design, grasp the research direction and innovation. He mainly focuses on the construction of artificial consciousness algorithm framework and team coordination management, and participates in the research of the integration mechanism of consciousness model and brain-computer system.
Artificial Intelligence and Algorithm Team: Led by Professor Zhang from the School of Computer Science of a university, including 3-4 assistant researchers and doctoral students. Responsible for the software implementation of the DIKWP decision engine, reinforcement learning algorithm integration, and semantic decoding model training optimization. Prof. Zhang has been engaged in brain-computer signal processing and machine learning for a long time, and the team will complete algorithm development and simulation experiments under the guidance of Prof. Duan to ensure that the artificial consciousness model runs efficiently and reliably in a closed-loop system.
Neural Engineering and Chip Team: Led by researcher Li from the Microelectronics Center of a research institute, including 2 chip engineers and 2 hardware engineers. Responsible for the design and implementation of artificial awareness chips (ACPU), electrode interface development and system integration. Prof. Li has a number of experience in the development of neuromodulation devices, and will lead the team to overcome engineering difficulties such as low-power chip circuits, implantable packaging, and wireless communication, and build a complete hardware platform.
Neuroscience and Animal Experiment Team: It is led by Professor Wang of a brain science research institute, including 2 postdoctoral fellows in neurobiology and several experimental technicians. Undertake the establishment of animal models of stroke and epilepsy, behavioral assessment and neurobiological testing. Professor Wang's team will obtain key experimental data to verify the biological effects of closed-loop interventions and provide a basis for algorithmic models. The team is also responsible for some in vitro brain tissue experiments to explore mechanisms at the synaptic and network levels.
Clinical Medical Team: Led by Chief Physician Liu of the Department of Neurology of the Affiliated Hospital, doctors from the Department of Rehabilitation and the Epilepsy Center. Responsible for clinical needs analysis and trial guidance, including patient recruitment, development of rehabilitation training programs, and monitoring of medical safety. Director Liu has rich experience in stroke rehabilitation and epilepsy treatment, and will ensure that the research protocol is in line with clinical practice, and will be responsible for the implementation and evaluation of the initial clinical trial in the later stage, helping the team to promote the scientific research results to the clinic.
Project Management & Ethics Consulting: Chen was hired as the project manager to coordinate the progress and resources of each unit, and was responsible for fund management, intellectual property declaration and external liaison. At the same time, one medical ethics expert consultant will be invited to guide the ethical approval and risk control of animal and human experiments throughout the process to ensure that the research meets the norms and ethical requirements.
Each sub-team complements each other in terms of personnel composition and is closely connected in terms of work content. In terms of specific responsibilities, the algorithm team and the neuroscience team work together to integrate experimental data into the model and continuously improve the algorithm; The chip team works with the algorithm team to transition the software algorithm to the hardware implementation; The clinical team provides demand traction and testing scenarios to verify the effectiveness of the system; Managers ensure smooth communication and adequate resources. Hold regular project team meetings (once a month, once a quarter, once a general meeting), and the person in charge will coordinate and discuss and solve cross-disciplinary problems. Through the above mechanisms, the project is ensured to advance with high quality as planned and produce the expected results.
Risk assessment and response strategies
This project has a high degree of cross-innovation, involving biology, engineering, AI and other aspects, and there are certain technical and implementation risks. We have assessed the key risk points and developed the following response strategies:
**Technical risk 1: Difficulty in decoding and semantic brain signals. **Brain signals are noisy and vary from person to person, making it challenging to convert massive amounts of neural data into explicit semantic information. Strategy: Multi-channel and multi-modal fusion are introduced to improve the signal-to-noise ratio, and the combination of advanced signal processing and deep learning is used to extract robust features. At the same time, an individualized baseline is established in advance: the resting and task status data of each subject are collected to train a customized decoding model and improve the recognition accuracy of gesture purpose. If it is still not ideal, we reserve the solution to simplify the semantic decoding requirements and extract a small number of key biomarker signals (such as specific band power) to ensure the operation of the basic functions of the system and buy time for subsequent optimization.
**Technical risk 2: Insufficient stability and real-time performance of closed-loop control. **Artificial consciousness algorithms need to parse brain signals and output stimulus decisions in milliseconds. Response lag or algorithm divergence may occur in complex environments. Solution: Algorithm optimization and hardware acceleration are used to ensure real-time performance. On the one hand, the model calculation is simplified, and the parallel computing characteristics of FPGA/ASIC are used to realize the pipeline processing of key steps. On the other hand, stability constraints are introduced: the restriction on the rate of change of the decision and the smoothing filter are added to the algorithm to avoid large output oscillations. Metacognitive monitoring acts as a guarantee, and if multiple consecutive decision instabilities are detected, the closed-loop gain will be automatically reduced or temporarily switched to safe mode. In addition, parameters are adjusted during the simulation and animal testing phases to ensure that the system is stable and fast before human testing.
**Scientific risks: The mechanism of brain plasticity is not obvious or the results are not as expected. **The plasticity effects induced by brain-computer interfaces are complex, and significant changes may be difficult to observe in a limited time. Coping strategy: Expand the sample size and observation indicators, and verify the plastic change by multiple means. For example, adding histological assays (e.g., synaptic protein expression) in animal experiments, extending follow-up time in human experiments, and improving detection capabilities with highly sensitive EEG/imaging indicators. Criteria should also be developed: if the primary hypothesis (e.g., closed-loop training promotes enhanced functional connectivity) is not validated, we will adjust the experimental parameters (e.g., increase the training frequency, change the stimulation pattern), or re-analyze for different patient subgroups to find a more pronounced pattern of effect. Since we are studying two types of diseases (stroke and epilepsy) at the same time, when the results in one direction are not good, the findings in the other direction can still support the value of the project, and we will also summarize the negative results and refine the lessons learned.
**Engineering risk: the development of artificial consciousness chips is delayed or the performance is not up to standard. **The design and manufacture of special chips are complex, and problems such as tape-out failure and excessive power consumption may be encountered. Strategy: Use the FPGA platform as a transition, and use the FPGA debug function while designing the ASIC. Even if the chip is delayed, we can use an external FPGA+ computer to simulate the chip function in the short term, so as to ensure that other work does not stop. In addition, we cooperate with experienced chip companies to conduct small-scale tape-out tests and module-level verification in advance to put risks in advance. For power consumption and heat generation, we design with sufficient redundancy, such as adding a heat sink to the package and supporting dynamic frequency adjustment in the algorithm, to ensure that even if the performance is slightly lower than expected, the system will not be seriously affected.
**Experiments and ethical risks: Accidents in animal or human trials. **Implantable electrodes can cause infection or tissue damage, and closed-loop stimulation has the potential to induce unintended responses. Coping Strategies: Adhere to the principle of ethical priority and gradual progress. In the animal experiment stage, sterile surgery and postoperative care should be carried out in strict accordance with the requirements of animal ethics, and an observation period should be set up to assess safety before proceeding to the next step. Human trials are conducted only after adequate animal validation, safety evaluation, and ethics committee approval, and initially only in a very small number of critically ill patients, in hospital ICUs or custodial settings, so that adverse events can be managed at any time. Establish a detailed emergency plan in advance, including guidelines for emergency stop (e.g., immediate shutdown of the device in the event of persistent seizures or abnormal heart rhythms), medical procedures, and division of responsibilities. In the event of any accidents, start planning immediately to minimize risks and injuries. The project also sets up an independent supervisory committee to regularly review the safety data of the trial, and recommend adjusting or discontinuing the trial if necessary to ensure that the study does not deviate from the ethical bottom line.
**People and management risk: Poor collaboration or personnel turnover in interdisciplinary teams. **The team involves multiple units and fields, and poor communication may delay progress; Changes in key personnel can also have a shock. Coping strategies: Establish an efficient project management system, communicate and report progress regularly, and use project management software to share information and plans. The project manager coordinates the schedule and identifies coordination problems in a timely manner. For key technical positions, assign deputies or echelons, for example, at least two people in each sub-project to master the core technology, so as to prevent work continuity when personnel leave their posts. Through regular training and brainstorming, the team understands the understanding of the team, so that the engineers understand some medicine, and the doctors also understand the basics of algorithms, so as to reduce communication barriers. Provide competitive support for key personnel in terms of funds, enhance team cohesion, and strive to maintain stability of core members during the project cycle.
Overall, we have fully identified the challenges that may be encountered in the project and prepared a multi-layered response. Relying on the advantages of multi-disciplinary and pre-research foundation, I believe that various risks can be reduced to a controllable range and the project tasks can be successfully completed.
Budget structure and description of funding
The total amount of funds to be applied for this project is XXX million yuan, which will be reasonably allocated to various expenditure subjects according to the needs of the research task. The budget structure and description are as follows:
Equipment cost (about 40%): Acquisition and development of key equipment for experimental and system development. Such as high-density EEG acquisition system, neurostimulator prototype, FPGA development board and simulation software, brain imaging equipment usage fee, etc. In addition, it includes the cost of tape-out of artificial consciousness chips (multiple trial production), the procurement of implantable electrodes and packaging materials, etc. Exoskeleton rehabilitation robots and epilepsy model animal monitoring equipment required for stroke rehabilitation training are also listed. This expenditure accounts for a relatively high proportion to ensure the smooth progress of hardware development and experiments.
Material consumables (about 10%): including biological experiment consumables (purchase and feeding of experimental animals, reagent testing, cell/tissue testing kits, etc.), as well as development consumables such as electronic components, PCB board production, and 3D printing shells. Due to the variety of materials involved in cross-disciplinary experiments, we refine the budget according to the needs of each task to ensure that it is sufficient and not wasted.
Personnel fee (about 20%): Labor allowance for full-time participants such as research assistants and postdoctoral fellows in the project team, as well as student research assistantship allowance. Personnel expenses are in line with relevant national standards and are used to motivate team members to invest in research. At the same time, a certain amount of funds are set aside for team training and travel (see below).
Testing and commissioning fees (about 5%): Some tests need to be commissioned to professional institutions, such as brain tissue slices and pathological analysis, large-scale microarray testing, and possibly human image data analysis. In addition, the project may need to outsource some software development or professional consulting services (e.g., medical device registration consulting), which are covered by this account.
Travel and international cooperation and exchange expenses (about 5%): used for the travel expenses of the research group members to participate in domestic and foreign academic conferences, technical training, and exchange visits with cooperative units (including the international artificial consciousness research team). This project is a cutting-edge interdisciplinary research, and it is necessary to go out to learn the latest progress, and consultants will also be invited to visit and guide. Funds will be used in strict accordance with the standards to support the smooth development of the project and the dissemination of results.
Management and other expenses (about 5%): including project management fees, paper layout fees, patent application fees, and equipment operation and maintenance fees. In particular, the power consumption, network and maintenance costs of the long-term operation of the system need to be taken into account. In addition, the management fee drawn according to the requirements of the relying unit is also included (if there is an upper limit, it will be withdrawn according to the regulations).
Reserve (about 5%): Establish an appropriate percentage of flexible funds to cover unforeseen expenses or price fluctuations. Such as new research opportunities, experimental failures that need to be repeated, key equipment updates, etc. The use of the reserve fee will be strictly approved to ensure that the funds are earmarked.
The above budgeting takes into account the breadth and depth of the study, and seeks to cover all necessary costs and maintain a balance across the various subjects. The focus of capital investment is inclined to the development of hardware equipment and core technology research, which is the material basis for project innovation. Personnel funds are also fully supported to attract and stabilize high-level talents. The proportion of each expenditure will be implemented in detail according to the actual progress, and we will use the funds in strict accordance with the management measures for scientific research funds to improve the efficiency of the use of funds. It is expected that through the reasonable investment of the project funds, high-value innovation results can be produced and value for money.
Expected outcomes and societal value
After three years of research, the project will achieve a series of innovative results in scientific research and technology application, which are expected as follows:
Theoretical innovations: Elucidate the mechanism of brain-computer interface-induced brain plasticity, and propose and verify the theoretical framework of artificial consciousness intervention to promote brain network reconstruction for the first time in the world. It is expected to publish 5~8 high-level academic papers, including about 2 in the field of neuroscience and about 2 in top journals of artificial intelligence. This paper will report in detail the effects and mechanisms of closed-loop interventions in stroke and epilepsy models, and the principles of DIKWP artificial consciousness algorithms, laying a new theoretical foundation for this interdisciplinary field. At the same time, we plan to give a presentation at the International Conference on Artificial Intelligence and Brain Science, and launch the theoretical concept of "DIKWP Consciousness Closed Loop" to enhance academic influence.
Key technical standards and evaluation indicators: Formulate technical specifications and effect evaluation standards for closed-loop brain-computer interface cognitive feedback training. For example, a set of evaluation indicators has been established in stroke rehabilitation, including the improvement rate of motor function scales and the rate of brain network connection enhancement, to measure the efficacy of intelligent BCI therapy. In epilepsy intervention, new indicators, such as the synchronicity reduction index of seizure network, were proposed to evaluate the improvement of brain network brought about by closed-loop intervention. These indicators and evaluation methods are expected to be incorporated into industry guidelines or standard documents to fill the gap in the evaluation of the effect of intelligent closed-loop neuromodulation. We will work with industry associations to promote standardization later in the project.
Invention patents and intellectual property rights: Focusing on the application of DIKWP artificial consciousness in brain-computer interface, we expect to apply for no less than 10 invention patents. The key points include: artificial consciousness chip architecture design, electrode parameter semantic plasticity control method, metacognitive security monitoring mechanism, etc. These patents will build a complete pool of independent intellectual property rights and provide legal protection for future productization. In consideration of international competition, we will also apply for PCT patents and strive to have a presence in major markets around the world. The patent achievements condense the core innovation of the project, which can establish a first-mover advantage for China in the field of intelligent brain-computer integration.
Prototype system and demonstration application: Delivered 1 set of bidirectional closed-loop brain-computer interface prototype system, including implantable hardware and supporting software. The system achieves at least TRL (Technology Maturity) level 4-5, i.e. key functions have been validated in the lab and in a simulation environment. We will demonstrate the application in the rehabilitation center and epilepsy center: show the paralyzed patients re-realize simple limb movements with the help of the system, and the epilepsy patients can significantly reduce their seizures through the system. A demonstration GUI interface will also be developed to display the artificial consciousness decision-making process (data, information, knowledge, wisdom, purpose) in real time for scientific research teaching and popular science. This prototype will be used as a prototype for subsequent clinical product development, and its component modules (such as artificial consciousness chips) can also be transplanted to other biomedical devices, which has important extended application value.
Talent training and team building: During the implementation of the project, more than 10 interdisciplinary talents will be cultivated. It is expected to complete the training of 3-4 doctoral and postdoctoral fellows, who will master the cross-skills of brain-computer interface, artificial intelligence and neuroscience, and become a new force in this field in China. The team is expected to further cohesion into a continuous innovation team through cooperation, and continue to tackle key problems together after the end of the project, so that the supporting unit can maintain an international leading position in the direction of the integration of artificial consciousness and brain science.
Socio-economic value: From the perspective of social benefits, this project provides new hope for rehabilitation and treatment for stroke and refractory epilepsy patients. If the results are transformed and applied, it will help thousands of stroke patients who have lost their ability to exercise to recover their independence faster and better, and reduce the burden on their families and society. It can also reduce seizures, improve quality of life for epilepsy patients, and reduce accidental injuries and medical expenses caused by seizures. Its potential social value is immeasurable. In terms of economic benefits, intelligent brain-computer rehabilitation and neuromodulation equipment are hot spots in emerging industries, and the global market space is broad. Once our team's technology matures, it can incubate high-tech enterprises and form an industrial chain from chips to medical devices. With the original advantages of DIKWP artificial awareness, our products will be internationally competitive and are expected to occupy a certain share of the high-end rehabilitation medical device market and generate considerable economic returns. More importantly, the project has achieved a breakthrough in the implementation of artificial intelligence technology in the field of medical and health care, responding to the dual needs of the "Healthy China" strategy and the development of digital technology, and demonstrating the huge potential of artificial intelligence to empower medical care.
In summary, this project will produce a wealth of results, from scientific theory to practical system, with clear layers and mutual support. These achievements not only push the academic frontier, but also effectively serve major clinical needs. We believe that the successful implementation of the project will establish China's international leading position in the field of artificial consciousness and brain-computer interface integration, bring tangible benefits to patients, and have a far-reaching impact on the development of related industries and society.
